JACOB BECKHAM - LSU BIOLOGICAL ENGINEERING
Project Summary
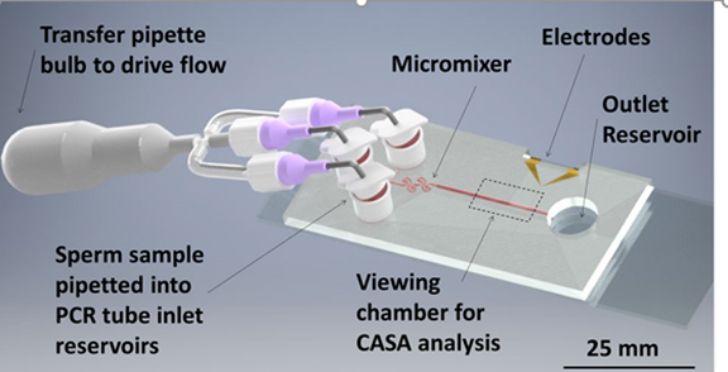
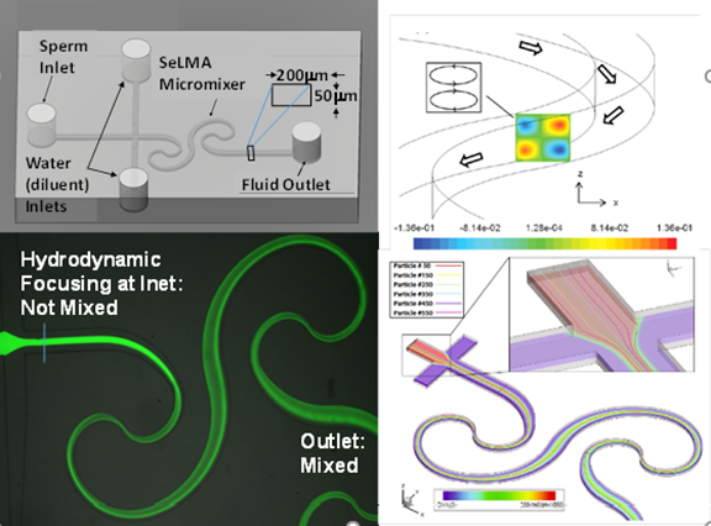
Sperm motility analysis is the current standard for determining sample quality. However, motility analysis of zebrafish sperm is difficult due to the small sample yield for each fish, small cell size, and short motility duration (10–15 s peak initial motility). For externally fertilizing freshwater species such as zebrafish, motility is initiated by introducing the sperm to a hypo-osmotic environment. This drop in osmolality causes the initially dormant sperm to become active. The time lag between activation and analysis, along with inconsistencies in activation due to human variation, are limitations in measuring peak motility of fish sperm, which can result in error-prone evaluation of sperm sample quality. Using lab-on-a-chip technology, a Microfabricated Activation & Motility Chamber (MAMC)(Fig. 1) was developed to facilitat research and quality control analysis of zebrafish sperm which, due to its miniscule (i.e., 2–5 μl) sample volume and short duration of motility (i.e., <1 min), present a challenge for traditional manual assessment methods. A micromixer (Fig. 2) molded in polydimethylsiloxane (PDMS) bonded to a glass substrate was used to activate sperm samples by mixing with water, initiated by the user depressing a transfer pipette connected to the chip. Sample flow in the microfluidic viewing chamber was able to be halted within 1 s, allowing for rapid analysis of the sample using established computer-assisted sperm analysis (CASA) methods. Zebrafish sperm cell activation was consistent with manual hand mixing and yielded higher values of motility at earlier time points, as well as more subtle time-dependent trends in motility, than those processed by hand. This device represents a pivotal step in streamlining methods for consistent, rapid assessment of sperm quality for aquatic species. The capability to rapidly activate sperm and consistently measure motility with CASA using the microfluidic device described herein will help improve the reproducibility of studies on sperm and assist development of germplasm repositories.