
Lucia Arregui, Phd
Postdoctoral Researcher
Project Summary
The clawed frogs (Xenopus laevis and X. tropicalis) are valuable biomedical models. Production of transgenic and mutant lines in these species has led to a rapidly expanding need for a cost-effective and efficient way to maintain the increasing number of lines at the National stock center for Xenopus (National Xenopus Resource, NXR, Woods Hole, Massachusetts). Storage of cryopreserved germplasm in repositories can provide a way to protect such lines and reduce the number of live animals held at NXR. In collaboration with NXR we are developing a sperm cryopreservation user-centered pathway (combination of a protocol and its practical application) for Xenopus sp. for the establishment of a germplasm repository.

SPERM QUALITY EVALUATION
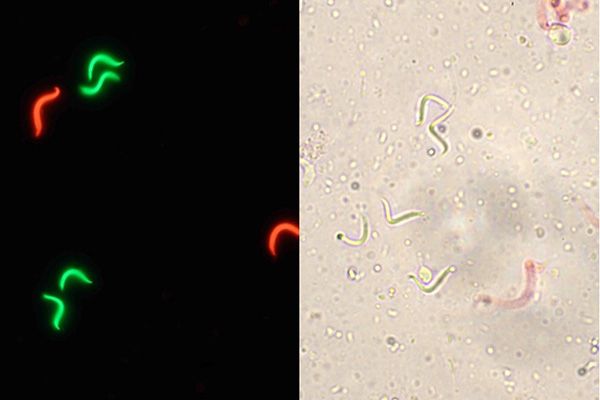
Cryopreservation is a multi-step process that can produce a significant loss on sperm quality. The first step for the development of a sperm cryopreservation protocol is to establish methods for quality evaluation of the sperm along the process of cryopreservation. For sperm, several parameters have been used for the evaluation of quality but the most common ones are motility and cell membrane integrity. We are standardizing for Xenopus a method for evaluation of motility using computer-assisted sperm analysis (CASA) as it allows performing a rapid, repeatable and accurate analysis. Membrane integrity is being quantified and compared using fluorescent (SYBR-14/PI) and non-fluorescent dyes (eosin Y) to open the opportunity to be performed using fluorescence or bright-field microscopy.

SPERM CRYOPRESERVATION PATHWAY
Once sperm quality could be assessed, we proceed to develop a cryopreservation protocol. We perform cryotoxicity test to select the less detrimental cryoprotectants and theirs concentration. Next, we evaluate each step of the cryopreservation process in relation to others. We assess the effect of sperm concentration, composition of cryodiluent and rate of freezing and thawing. Finally, we test fertilization capacity of sperm samples cryopreserved using the most suitable protocols. We are considering the application of this protocol together with the protocol itself. Therefore, we integrate many factors besides cryopreservation itself for the development of this pathway such as cost, labelling, simplicity, scalability or database requirements.
OPTIMIZING SPERM CRYOPRESERVATION PATHWAY
Multiple factors before, during and after freezing and thawing will be analyzed to consider its effect during the cryopreservation process. The role of temperature of animal housing and animal age on gamete quality will be determined. Also, sperm samples are collected through maceration of the testes and different methods of testes preparation (tearing with forceps, grinding with pestle) will be analyzed to assess the effect on sperm quality and freezability.

Team Members

Rose Upton - Postdoctoral Researcher

Luke Turner - Undergraduate Student Worker

Lily Carter - Undergraduate Student Worker
Collaborators

Marko Horb - Director, The National Xenopus Resource

Nikko-Ideen Shaidani - Manager, The National Xenopus Resource

James Parente - Assistant manager, The National Xenopus Resource

Carl Anderson - Research Assistant, The National Xenopus Resource
Funding Organizations
